Weill Cornell Medicine (WCM) activities shape the direction of future FDA device surveillance system development. The infrastructure and methods that we continue to develop are viewed as the bases for real-time, active surveillance, allowing consideration of expanding indications for devices, contributing to harm and benefit balance assessment, leading to less costly post-approval studies and advancing the unique, clinically meaningful device identification process. The regulatory-scientific FDA figure below is developed based on WCM Medical Device Epidemiology Network (MDEpiNet) research success.
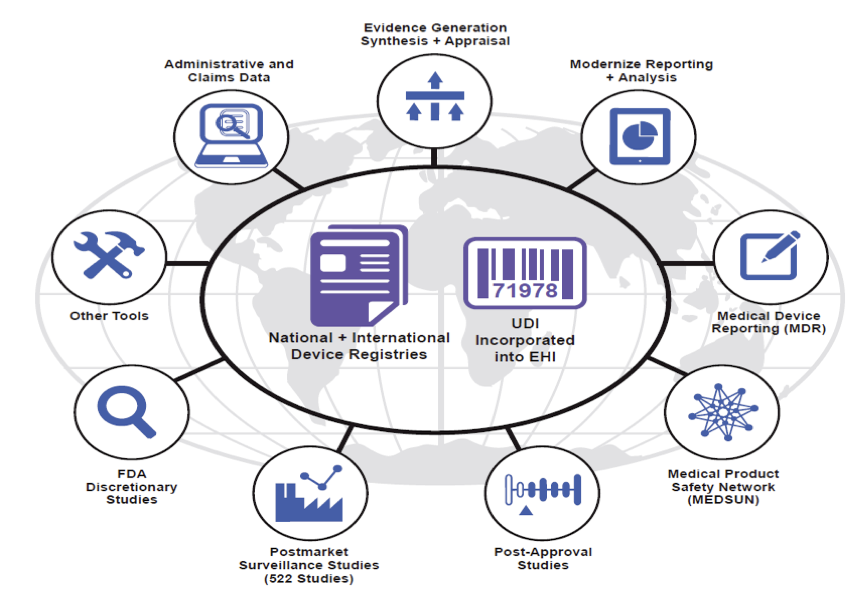
Regulatory vision of surveillance development for medical device safety and effectiveness
International Medical Device Regulators Forum (IMDRF) Registry Working Group
The IMDRF was conceived in February 2011 as a forum to discuss future directions in medical device regulatory harmonization. The IMDRF's voluntary group of medical device regulators from around the world come together to build on the strong foundational work of the Global Harmonization Task Force (GHTF) and accelerate international medical device regulatory harmonization and convergence. WCM representatives lead the IMDRF Registry Working Group, with the purpose of developing:
- Essential principles related to registries, international collaborations and linkage of registries with electronic patient, device and outcome data repositories or identifiers, including the principles of data access, security, informatics formats, governance and other key areas related to global regulatory applications for medical device evaluation.
- Essential principles related to optimal methodologies for analysis of heterogeneous data sources applied to medical device safety, signal detection, performance and reliability.
Additionally, we provide specialistic advice for the Australian Therapeutic Goods Administration (TGA).

