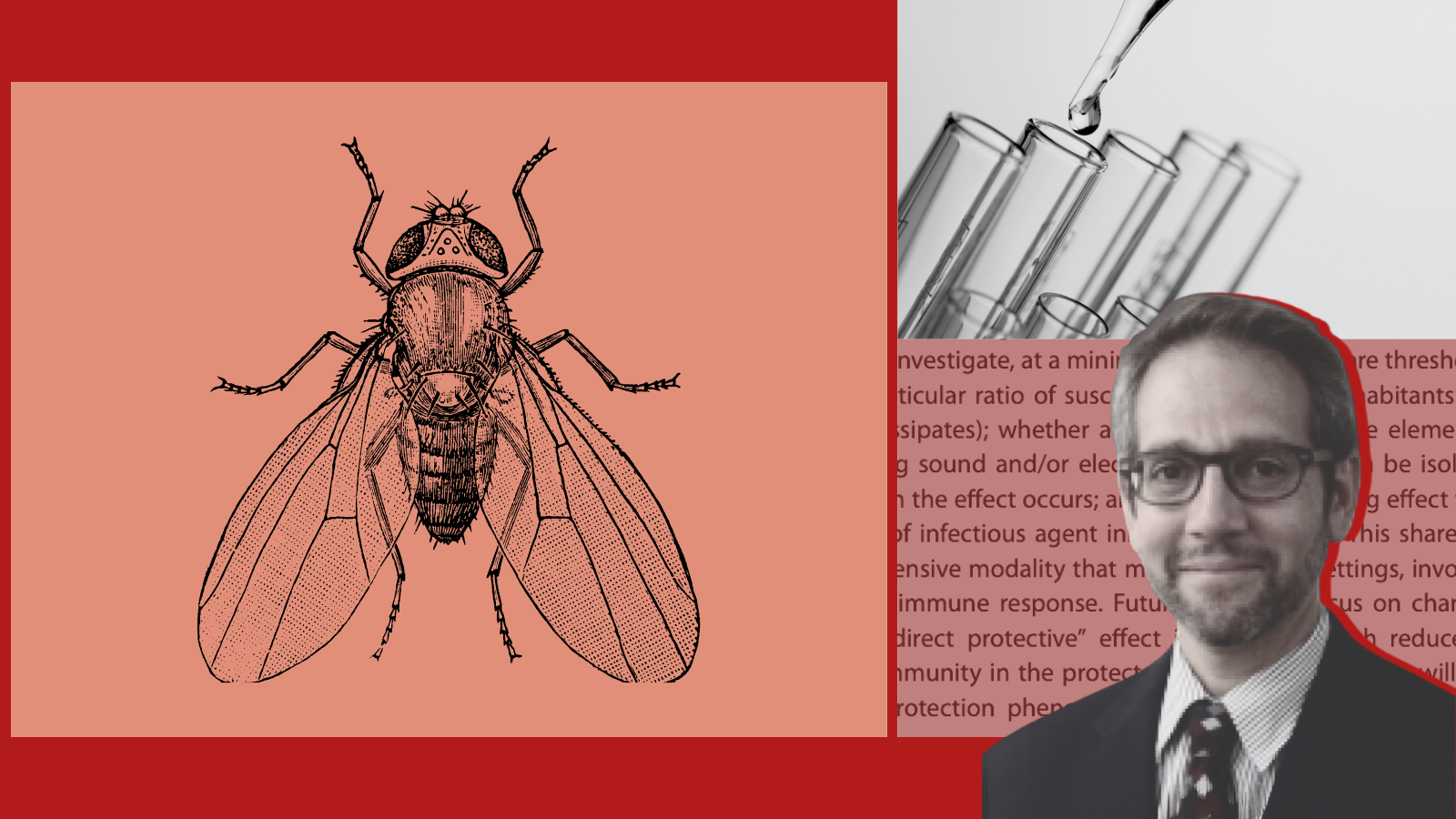
The humble fruit fly Drosophila melanogaster (Dm), which has been one of the key animal models underpinning modern medical research for over 100 years, still has some surprises up its sleeve. Flies and other organisms are known to have diverse sensing strategies to identify and initiate immunological defenses against noxious environmental elements. In a study published in mBio, the journal of the American Society for Microbiology, Dr. Nathaniel Hupert, associate professor of population health sciences and medicine, along with colleagues at the Feinstein Institutes for Medical Research, George Washington University, and Yale School of Medicine, found that Dm appears to share those defenses with their close neighbors, leading the researchers to coin the concept of “proximate” or “shared immunity.”
Their study, framed by questions about the relatively low rates of household transmission of COVID-19 combined with evidence that uninfected family members of COVID-19 cases show specific T-cell activation, a key event in the adaptive immune response, asked whether Dm could be protected from death simply by living in the same test tube with fellow flies whose immune systems were “primed” to fight infections. The work capitalized on previous studies in Dm showing that priming flies by infecting them with live Escherichia coli (Ec) confers protection against more serious subsequent infection, in this case with the highly lethal insect pathogen, Photorhabdus luminescens (Pl).
The experiments showed that 100 percent of the unprimed flies living only with other unprimed flies quickly died when exposed to Pl, but those co-habiting with primed flies were highly protected against infection. Whereas 80 percent of the primed flies survived the Pl challenge, 60 percent of their unprimed co-habitants were also protected. The researchers showed that this protection was not due to cross-infection with Ec, but the exact mechanism of shared protection remains unknown. The next phase of research will be to investigate possible modalities including both chemical (i.e., airborne or contact-related molecules) and physical (e.g., soundwave, electrical, or magnetic) mediators of protection.
These findings are the first to indicate that the presence of infected hosts can boost the immune response of healthy Dm flies in close proximity, leading to what researchers suggest is a shared immune collective. “The immune system is full of surprises, and this is another,” says Dr. Douglas Nixon, lead author of the study and Director of the Institute of Translational Research at the Feinstein Institutes for Medical Research. “Sharing of protection by proximity as we have shown here in flies, extends the reach with which an organism can protect itself against an invader. A solitary fly which joins a ‘herd’ is balancing risk of infection versus proximate protection, and future ecological and immunological studies will determine how vital this protection is to survival.”
The work may have important implications for both basic science and public health. “If borne out in other species, these results could give us a radically new understanding of the dynamics of disease transmission in households and other congregate settings,” notes Dr. Hupert. “For example, the implications of this proximate immunity concept for modeling of infectious diseases could be profound, since current models only take account of transmission risk, not potentially protective effects.”
- Highlights

